Bladder cancer is currently treated in its earlier stages by localized intravesical chemotherapy or immunotherapy after removing the tumor. However, recurrences occur in up to 70% of patients at 5 years, while up to 30% fail to respond. This means a constant need to monitor and retreat these patients, driving costs sky-high for the treatment of this form of cancer. A new study in Nature Nanotechnology explores the potential use of nanobots to enhance the therapeutic efficacy of intravesical treatment of bladder cancer.
Study: Urease-powered nanobots for radionuclide bladder cancer therapy
At present, for bladder cancers that have not reached the stage of muscle invasion, the tumor is removed, followed by the intravesical use of the immunotherapeutic agent Mycobacterium bovis Bacillus Calmette–Guérin (BCG) or the chemotherapeutic drug mitomycin C, singly or in combination. Issues with this mode of treatment include non-uniform dispersion throughout the bladder, rapid transit, and low adhesion of the agent to target tissue, leading to sedimentation within the bladder. All these are related to the constant arrival of fresh urine into the bladder.
The ideal agent should be able to enter tumor tissue to adequate depths, treat the whole tumor, and be compatible with human tissue. Nanobots are self-propelled nanoparticles that may be of immense utility in intravesical chemotherapy because they can diffuse and mix much better in body fluids like urine compared to conventional drug formulations or ordinary nanoparticles.
In the current study, the focus is on the ability of nanobots to improve the low therapeutic efficacy of intravesical bladder cancer therapy. The researchers used radiolabelled nanobots of 450 nm diameter built on a mesoporous silica base.
These were designed to propel themselves using chemical energy from reactions based on substrates in the surrounding fluid. In this case, urea split by the enzyme urease, carried on the nanobots.
The production of ammonia and CO2 around the particle during the reaction enhanced the motion of these particles. In particular, this drives a swarming behavior that is associated with higher convection and mixing and inhibition of sedimentation. This gives them immense potential as carriers for radionuclide therapy (RNT).
They were tested for therapeutic efficacy in bladder cancer in a mouse model.
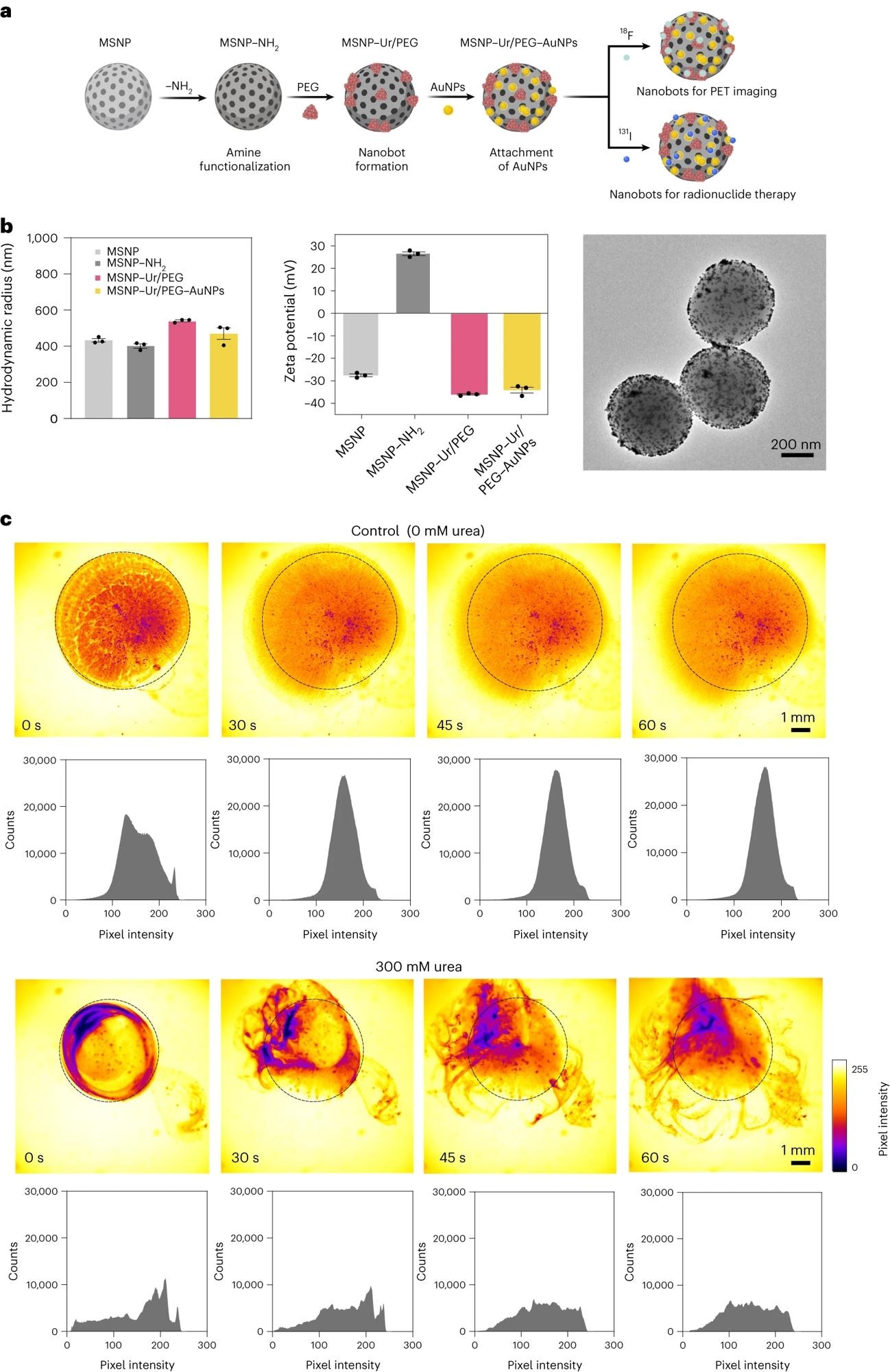
a, Schematic representation of the nanobot fabrication process and radiolabelling. Ur, urease. b, Left: nanobot characterization by dynamic light scattering (n = 3, technical replicates). Data are presented as mean values and error bars represent the s.e.m. Centre: zeta potential (n = 3, technical replicates) Data are presented as mean values and error bars represent the s.e.m. Right: transmission electron microscopy image. c, Snapshots depicting the nanobot motion dynamics in the absence and presence (300 mM) of urea as fuel, and corresponding pixel intensity histograms for the ROI marked by a circle. Panel a created with BioRender.com.
What did the study show?
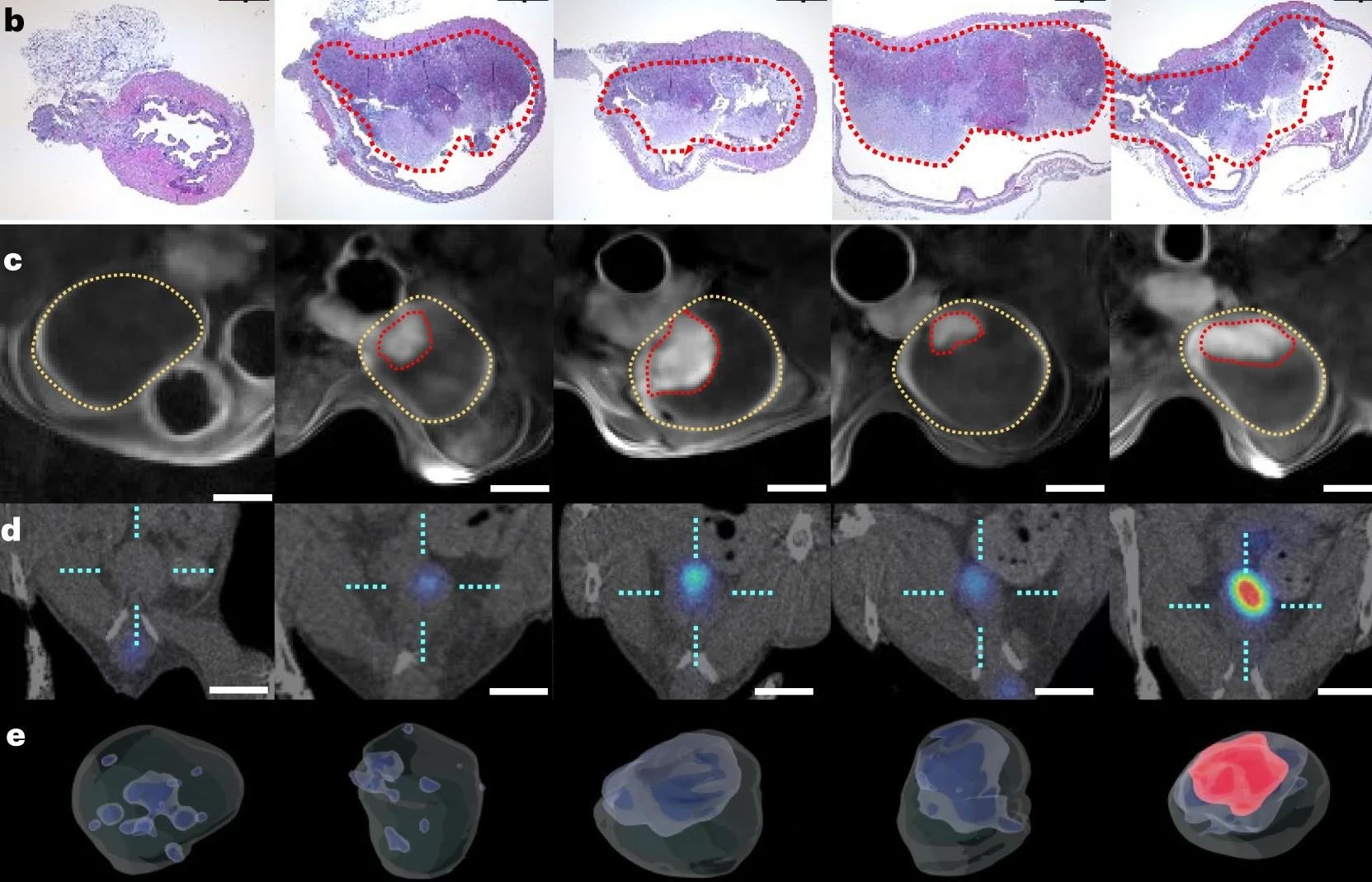
Both ex vivo and in vivo experiments showed that nanobots achieved a higher concentration at the tumor site, at 8-fold increased levels confirmed by positron emission tomography (PET). The presence of urea led to active dispersion of the particles with swarming coordinated motion.
Effective nanobot motion occurred only in the presence of urease and urea, and without enzymatic activity, tumor uptake of the labeled nanobots failed to occur.
Confirmation of activity was first sought using optimized optical contrast methods based on polarization-dependent scattered light-sheet microscopy. This showed that the tumors had been penetrated by the nanobots in ex vivo experiments. Conversely, little adherence or penetration occurred in healthy bladder tissue.
This method was also validated for in vivo observations of nanobots, as it offers high-grain resolution in a 3D format.
Unlike water, the urea-containing environment led to the successful self-propulsion of the nanobots to accumulate within the tumor tissue. This may be partly because the diseased bladder epithelium is more permeable to these particles by the breakdown of epithelial tight junctions. Also, the nanobots themselves may attack the tumor’s extracellular matrix (ECM) because of the basic ammonia produced by the urease-catalyzed reaction.
With the mouse model, when the mice were treated with nanobots carrying radioiodine for RNT instilled into the bladder, the tumors shrank by about 90%, even at low doses. Radioiodine is extensively used in RNT, being a beta-particle emitter with an 8-day half-life and capable of penetrating to a depth of 0.8 mm (about 0.03 in) into tissue.
This indicates that the active movement of the nanobots enhances tumor accumulation. This favors their clinical translation. Even at higher doses, the animals remained within standard weight limits, with an even greater fall in tumor volume.
“This suggests that 131I-carrying nanobots can effectively treat bladder tumors in confined spaces, presenting an alternative treatment for scenarios where traditional therapeutic approaches, such as BCG, routinely fail.”
What are the implications?
The results suggest urease-powered nanobots could be “efficient delivery nanosystems for bladder cancer therapy.”